- Biotech Snap
- Posts
- A wave of hearing loss gene therapies & Boehringer advance a kidney drug into pivotal trial
A wave of hearing loss gene therapies & Boehringer advance a kidney drug into pivotal trial

👉 Download your free copy of Lipotype’s latest Lipidomics eBook
Good morning! France’s Sensorion unveiled a €60m financing yesterday, including a €20m strategic investment from Sanofi. The financing pushes Sensorion’s cash runway into H1 2027 and will accelerate two gene therapy programs with regulatory filings, first patient enrollment, and Phase 1/2 readouts on deck, alongside Phase 2a data for its small-molecule SENS-401.
Yes, but: As you’ll see in our Snapshot below, Sensorion wasn’t the only European biotech to attract attention to its gene therapy pipeline for hearing loss yesterday.
Enjoy today’s read!
—Joachim E.
Don’t keep this newsletter a secret: Forward Biotech Snap to a friend!
Was this email forwarded to you? Sign up here.
SNAPSHOT
Seamless signs $1.1B Eli Lilly gene therapy deal to tune up hearing

Dresden-based Seamless Therapeutics has signed a global research and licensing deal with Eli Lilly worth up to $1.1 billion to develop gene therapies for genetic hearing loss using its proprietary recombinase technology.
Why it matters: The partnership validates Seamless’s novel gene editing approach and positions it as a key player in precision gene therapy, especially in an area with limited treatment options.
Backstory: Seamless developed a gene editing platform using engineered recombinases, enzymes that allow precise DNA changes without relying on cellular repair mechanisms, differentiating it from CRISPR/Cas.
Zoom in: Seamless will design site-specific recombinases to correct mutations in hearing-related genes. The platform enables high-precision DNA edits, including long sequence insertions, at specific genomic locations. While still preclinical, animal study data helped secure the Lilly deal.
Big picture: This deal signals rising pharma interest in next-gen gene editing tools beyond CRISPR and highlights the growing focus on treating genetic hearing loss, a condition affecting millions globally. The fact that Sensorion raised €60M the same day with the support of Sanofi (see our intro) makes this even more apparent.
PRESENTED BY LIPOTYPE
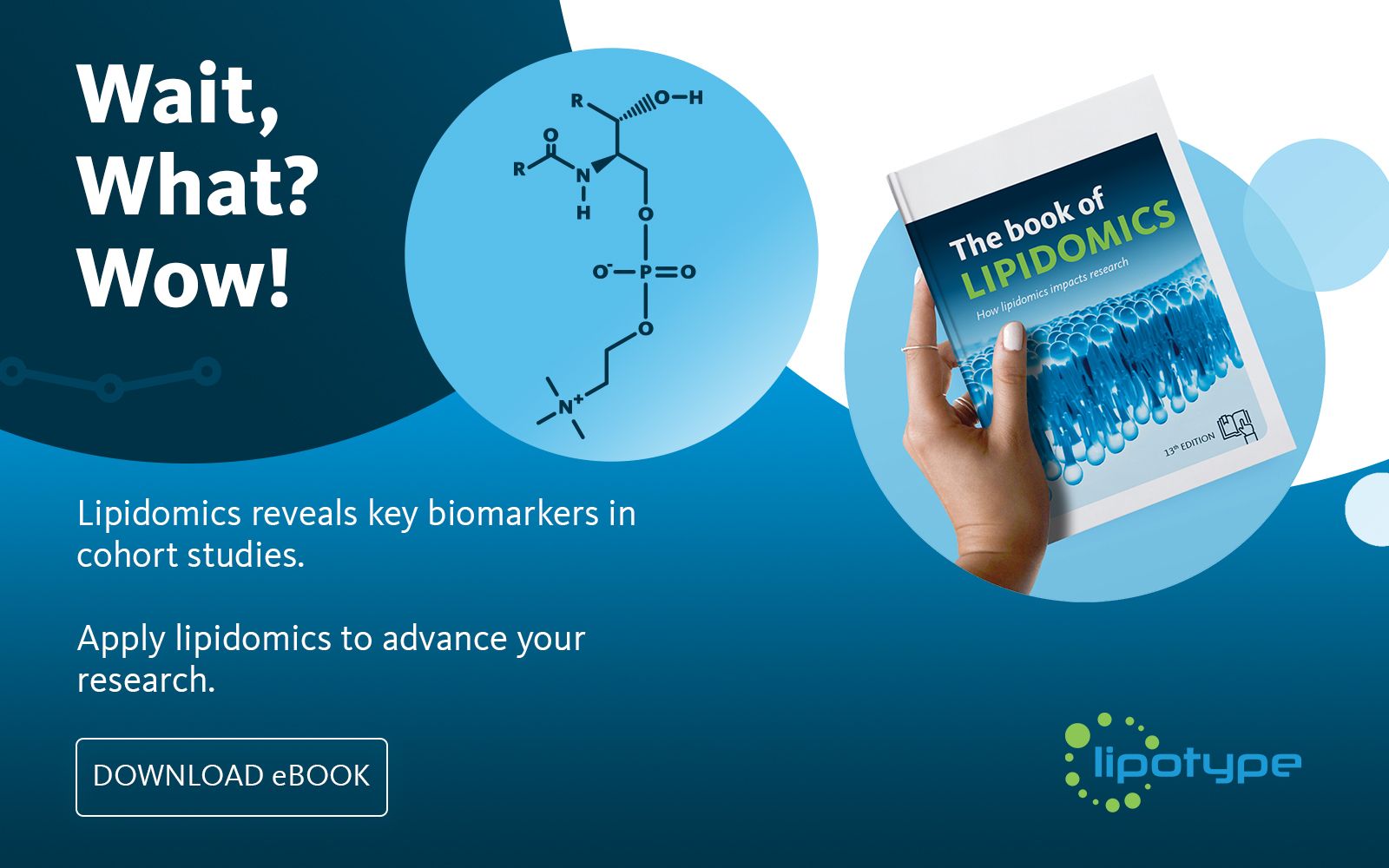
Learn how your project can benefit from lipidomics
Lipidomics is a field of research that analyzes lipids across diverse biological samples, focusing on their roles, pathways, and networks to explore biological mechanisms.
Lipotype’s Lipidomics eBook features several case studies that demonstrate how lipidomics can advance research across various fields, including neuroscience, dermatology, oncology, immunology, metabolic disorders, and more.
SNIPPETS
What’s happening in biotech today?
🧠 Tumor trouble: The FDA has placed a clinical hold on two of Regenxbio’s gene therapies, RGX-111 for Hurler syndrome and RGX-121 for Hunter syndrome, after a brain tumor was detected in a child who had received RGX-111 four years prior. While no causal link has been established, the FDA extended the hold to RGX-121 due to similarities between the therapies. The suspension comes just weeks before RGX-121’s expected approval decision, surprising both the company and analysts. Despite years of safety data and no similar cases among other participants, the incident highlights ongoing safety concerns in gene therapy development and regulatory unpredictability under current FDA leadership.
🌊 Surfing the wave: Halozyme Therapeutics announced the acquisition of privately held Surf Bio for $300 million upfront, with the total deal potentially reaching $400 million, alongside its financial guidance update. Surf Bio specializes in a drug delivery technology that enables rapid, high-concentration subcutaneous injections across various therapeutic areas. Halozyme stated the acquisition aligns with its strategic goals and enhances its long-term growth potential.
💸 Derm dollars: TRex Bio has secured a $50 million Series B extension to advance clinical testing of its lead drug candidate, TRB-061, for atopic dermatitis. The drug, currently in Phase 1 trials, targets the TNFR2 protein to activate regulatory T cells (Tregs) and control inflammation without broadly suppressing the immune system. The company plans to begin early human trials for two additional immune disease treatments, TRB-071 and TRB-081, next year. Despite competition from other biotechs and pharma companies, TRex aims to offer a more precise approach to immune regulation.
🚨 Kidney crisis: CalciMedica’s stock plunged 84% after the company halted its Phase 2 Kourage trial of Auxora, a CRAC channel inhibitor for acute kidney injury (AKI) with acute hypoxemic respiratory failure, due to a safety concern identified by an independent data monitoring committee. While no deaths or serious adverse events were linked to the drug, the company suspended the trial to review unblinded data and consider modifying its design and enrollment criteria. Despite this setback, CalciMedica emphasized Auxora’s clean safety record in over 350 patients across other trials and plans to continue development in acute pancreatitis and other inflammatory conditions.
🧬 Splice & dice: Akari Therapeutics has filed a new U.S. provisional patent for AKTX-102, its second antibody-drug conjugate (ADC) targeting CEACAM5, a tumor marker found in multiple high-need cancers such as colorectal, pancreatic, bladder, lung, and certain breast cancers. AKTX-102 combines a CEACAM5-targeting antibody with Akari’s proprietary PH1 spliceosome-modulating payload, aiming to enhance tumor cell killing and stimulate immune responses. The program has the potential to address aggressive cancers, including KRAS-mutated types.
SNAP AGAIN
Promising Boehringer midstage data helps the company advance kidney drug into pivotal trial

Boehringer Ingelheim is launching a phase 3 trial of its kidney drug apecotrep after phase 2 data showed it reduced proteinuria in focal segmental glomerulosclerosis (FSGS) patients, a disease with no FDA-approved treatments.
Why it matters: FSGS is a serious kidney disorder with limited therapeutic options. Apecotrep works by blocking TRPC6 overactivity, which may slow kidney decline. Apecotrep’s progression to late-stage testing adds momentum in the race to bring a treatment to market.
Backstory: In a phase 2 study, 35% of patients receiving apecotrep saw reductions in proteinuria versus 7% on placebo. The drug targets TRPC6, an ion channel linked to damage in podocytes, key kidney cells impaired in FSGS.
Big picture: The FSGS treatment landscape is heating up, with major players like BioMarin (Phase 3), Sanofi (Phase 2), Novartis (Phase 2), Vertex (Phase 2), and Vera Therapeutics (Phase 2) also pursuing new therapies. Travere is the most advanced player and is currently awaiting an FDA ruling by April 13.
What’s next: Boehringer will enroll 286 FSGS patients in its phase 3 trial, measuring changes in urine protein-creatinine ratio over 104 weeks. The trial is expected to complete in 2029.
TOUR OPERATOR
Upcoming events
🇳🇱 Amsterdam, 3-4 March 2026 - BioCapital
🇪🇸 Barcelona, 10-12 March 2026 - Bioprocessing Summit Europe
🇳🇱 Utrecht, 26 March 2026 - Innovation for Health
🇦🇹 Vienna, 27-30 March 2026 - BioProcess International
🇩🇪 Munich, 17-21 April - ESCMID Global
🇺🇸 San Diego, 17-22 April 2026 - AACR Annual Meeting
🇩🇪 Leipzig, 21-22 April 2026 - German Biotech Days
🇨🇳 Shanghai, 28-29 April 2026 - ChinaBio
🇸🇦 Riyadh, 11-13 May 2026 - BIO Middle East
🇩🇪 Berlin, 09 – 11 June 2026 - bio:cap
What did you think of today's newsletter?Your feedback helps us create the best newsletter possible. |
Share the Snap!
Know someone who’d enjoy this? Hit forward and pass it along.